Electroplating

Electroplating
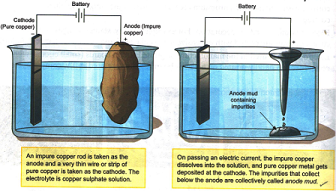
The method involved in electroplating is as follow:
1. The object to be coated is made the cathode.
2. The metal to be deposited on the object is taken in the form of an electrode and made the anode.
3. The electrolyte contains dissolved salts of the metal to be coated.
4. Ions of the metal (which are positively charged) are attracted by the cathode and therefore move towards the object and get deposited on it.
Electroplating is done for many purposes. Here are a few examples:
1. Metals that corrode easily are protected by coating them with a metal that does not corrode easily. Nickel and chromium are widely used in the automobile industry for coating.
2. Electroplating is used for decoration. For example, cutlery, statues, and jewellery made of cheaper metals are coated with expensive metals like gold and silver.
3. Electroplating is used in the manufacture of printed circuit boards. Which are used in T.V, computer, etc.
During electroplating, the electrolyte is made of _______________________ . | |||
| Right Option : C | |||
| View Explanation | |||
Which of the following are correct : (a) The object to be coated is made the cathode. (b) The object to be coated is made the anode. (c) The electrolyte contains dissolved salts of the metal to be coated. | |||
| Right Option : D | |||
| View Explanation | |||
Which of the following statements is/are correct : (a) The object to be coated is made the cathode. (b) The electrolyte contains dissolved salts of the metal to be coated.
| |||
| Right Option : C | |||
| View Explanation | |||
Students / Parents Reviews [10]
It was a good experience with Abhyas Academy. I even faced problems in starting but slowly and steadily overcomed. Especially reasoning classes helped me a lot.

Cheshta
10thAbhyas Methodology is very good. It is based on according to student and each child manages accordingly to its properly. Methodology has improved the abilities of students to shine them in future.

Manish Kumar
10thMy experience with Abhyas academy is very good. I did not think that my every subject coming here will be so strong. The main thing is that the online tests had made me learn here more things.

Hiya Gupta
8thMy experience with Abhyas is very good. I have learnt many things here like vedic maths and reasoning also. Teachers here first take our doubts and then there are assignments to verify our weak points.

Shivam Rana
7thA marvelous experience with Abhyas. I am glad to share that my ward has achieved more than enough at the Ambala ABHYAS centre. Years have passed on and more and more he has gained. May the centre flourish and develop day by day by the grace of God.

Archit Segal
7thIt was good as the experience because as we had come here we had been improved in a such envirnment created here.Extra is taught which is beneficial for future.

Eshan Arora
8thAbhyas is a complete education Institute. Here extreme care is taken by teacher with the help of regular exam. Extra classes also conducted by the institute, if the student is weak.

Om Umang
10thI have spent a wonderful time in Abhyas academy. It has made my reasoning more apt, English more stronger and Maths an interesting subject for me. It has given me a habbit of self studying

Yatharthi Sharma
10thMy experience was very good with Abhyas academy. I am studying here from 6th class and I am satisfied by its results in my life. I improved a lot here ahead of school syllabus.

Ayan Ghosh
8thIt has a great methodology. Students here can get analysis to their test quickly.We can learn easily through PPTs and the testing methods are good. We know that where we have to practice








